Electrophilic Reactivity Providing Well-Defined Helically Chiral Gold(III) Catalysts for the Asymmetric Synthesis of Bioactive Compounds
Hanock Baiju — Hector RCD Awardee Agnieszka Nowak-Król
Hector Fellow A. Stephen K. Hashmi
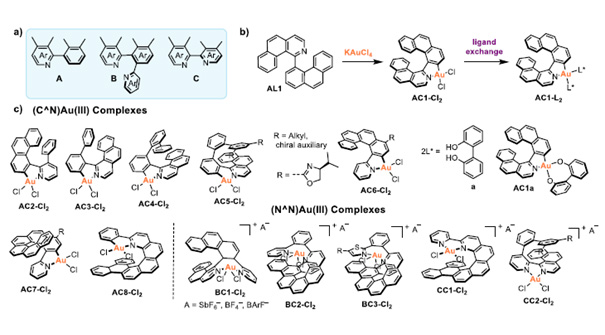
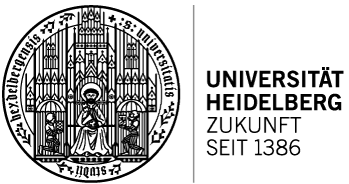
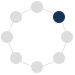
The aim of the project led by Hector RCD Awardee Agnieszka Nowak-Król (University of Würzburg) and Hector Fellow A. Stephen K. Hashmi (Heidelberg University) is to develop well-defined helically chiral gold(III) complexes, the first examples of helically chiral gold complexes with gold atoms on either an outer or an inner helicene rim. These compounds will be derived from axially chiral C^N and N^N chelate ligands by auration to form five- and seven-membered auracycles, thereby inducing helical frameworks. The catalytic potential of these unprecedented complexes and their practical utility will be demonstrated in the enantioselective synthesis of small organic compounds and biologically or pharmaceutically relevant targets, i.e. natural products and pharmaceutically active compounds.
Chirality plays a major role in life science and in drug design. The term chirality is defined as the potential of a molecule to exist in two distinct, non-superimposable forms that are mirror images of one another, wherein the atomic composition, atom-atom connections, and bond orders remain unaltered. Since enzymes and receptors in living organisms are also chiral, they can interact differently with both enantiomers, i.e. stereoisomers differing in the configuration of all stereogenic elements. Due to a different spatial arrangement of atoms in the molecule, one enantiomer can bind more strongly and with greater specificity to biological targets than the other enantiomer. These specific interactions determine the drug’s pharmacodynamics, pharmacokinetics, and toxicity. Thus, one form may induce a desired therapeutic effect, while the other remain inactive, show lower potency or cause adverse effects.
The achievements in gold catalysis over the last decades have established its place in organic chemistry. In general, gold catalysts operate efficiently under milder conditions than other transition metal catalysts, including lower temperatures, which can prevent the degradation of sensitive substrates. This is particularly beneficial in pharmaceutical synthesis, where maintaining the structural integrity is critical. In addition, gold-catalyzed transformations offer high atom economy and functional group tolerance, may exhibit orthogonal reactivity compared to other transition metal catalysts.
The objective of this project is the development of a new class of gold(III) complexes derived from helically chiral chelate ligands that will operate as catalysts in gold-mediated asymmetric transformations. The gold will be incorporated into the helical framework. Such complexes are to date unprecedented. The helicene structure will provide a well-defined, rigid chiral environment around the gold(III) center, creating a chiral pocket that can selectively interact with prochiral substrates. We expect that this will allow to achieve high enantioselectivity in gold(III)-mediated reactions. In addition, the π‑conjugated scaffold of a rationally designed aurahelicene can enhance the stereoselctive interactions with a substrate through non-covalent interactions, resulting in high chiral induction. These interactions can be further tuned by modifying the electronic properties of the helicene through the incorporation of selected heterocycles into the helical backbone or its selective functionalization with electron-accepting or electron-donating groups.
Figure: a) General motifs present in the target C^N and N^N ligands. Ar = six- or five-membered ring. b) Synthesis of a (C^N)Au(III) complex. c) Structures of selected target compounds.
Supervised by

Agnieszka Nowak-Król
Chemistry

A. Stephen K. Hashmi
ChemistryHector Fellow since 2010