Neuroimmune-vascular interplay in Alzheimer’s disease
Matteo Rovere – Hector Fellow Christian Haass
Alzheimer’s disease (AD) has a multifactorial etiology which includes, among others, vascular dysfunction and aberrant neuroimmunity. We aim to investigate the gene ABI3 as a potential connection between these two facets of AD pathophysiology. Through transgenic murine models, and using a combination of biochemical, immunohistochemical, and in vivo imaging techniques, we will explore how the late-onset AD risk variant S209F ABI3 affects neurodegeneration, immune fitness, and vascular dynamics.
Alzheimer's disease (AD) is the foremost cause of dementia and constitutes a major societal and economic burden. Despite recent breakthroughs, there is still no established therapeutic regimen capable of stopping or reversing it. Given its complex multifactorial nature, drug combinations hold the greatest potential to achieve these goals and, among others, vascular pathology and neuroimmune dysfunction are both promising targets still out of therapeutic reach.
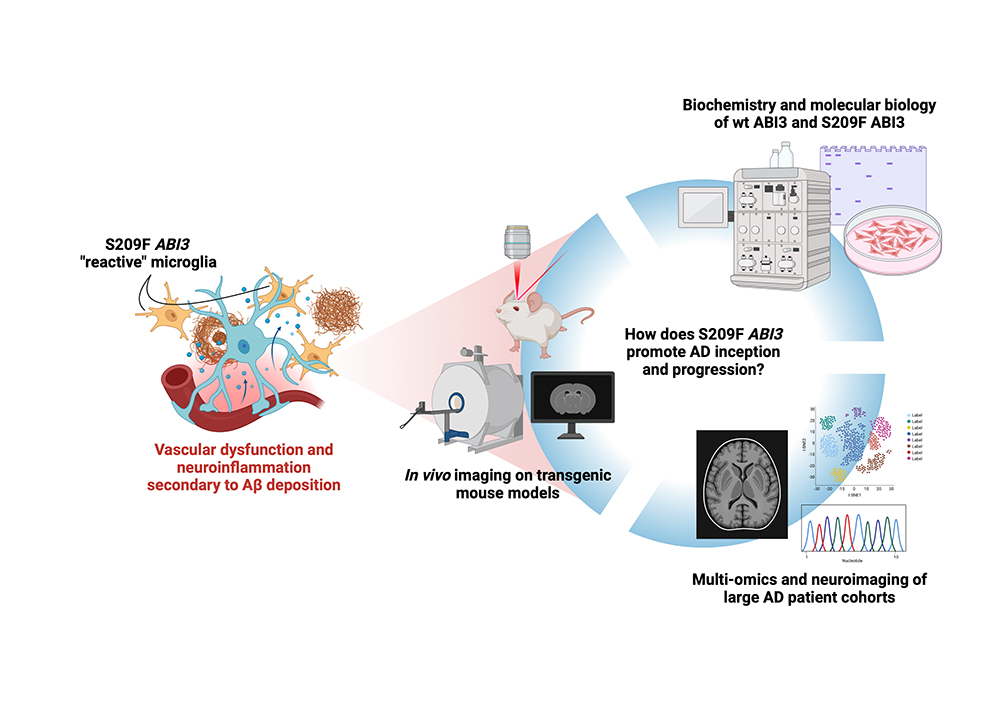
We are interested in the ABI3 gene, whose S209F variant carries an increased risk of developing late-onset AD. Preliminary work has shown that transgenic mice carrying the murine analog of S209F ABI3 present with microglial and neurovascular abnormalities, and we believe this could be one way through which these seemingly independent mechanisms of disease synergize. In this project we aim to characterize the biochemistry and molecular biology of ABI3 in health and disease and, through the use of transgenic AD murine models, detail its effects on the progression of neurodegeneration, neuroimmune responses, and vascular dynamics. We will employ a combination of biochemistry, immunohistochemistry on murine and human tissues, in vivo imaging of microglial motility and vascular responses, and multi-omics of large patient cohorts.
Overall, our work could not only help identify novel translational targets, but also inform the adoption of clinical interventions (e.g. vasoprotective measures) targeted at dementia patients.
S209F ABI3 knock-in and knockout transgenic mice exhibit neurovascular defects and microglial branching and motility changes. Our project aims to identify the molecular mechanism(s) behind these phenotypes through a combination of biochemistry and molecular biology, in vivo imaging on transgenic mouse models, and multi-omics and neuroimaging data collected on large AD patient cohorts.

Matteo Rovere
Ludwig-Maximilians-Universität MunichSupervised by

Christian Haass
Medicine