Magnetism in Perovskite Manganites and Cobaltites at the Nano Scale
Cornelia E. Hintze – Hector Fellow Hilbert von Löhneysen
The exact position of atoms in the crystal structure of lanthanum manganites and cobaltates (both anorganic ionic compounds) significantly affects their magnetic properties. The crystal structure of these materials can be altered by pressure, substitution of elements, or crystallite size: Since nanoparticles have a large surface-to-volume ratio, their surface has a dominant effect on the crystal structure, leading to changes compared to bulk materials. The variation in size can thus be used to induce variations in magnetic transition temperature and magnetic ordering.
Having produced nanoparticles of La1-xSrxMnO3 and LaCoO3 by microemulsion synthesis, it has been shown for La1-xSrxMnO3, that decreasing the nanoparticle size leads to an expansion of the unit cell, probably due to surface adsorbates. The elongation of the bond length results in a decrease of the magnetic transition temperature, whereby the mechanism is due to both reduction of double-exchange coupling and intrinsic size effect (see Figure). In LaCoO3, on the other hand, the elongation of the Co‑O bond length induces a change in the Co3+ state from low-spin to high-spin, in contrast to bulk LaCoO3 (low spin at low temperatures). In contrast to previously used preparation techniques for transition-metal-oxide nanoparticles, the microemulsion method allows control of the nanoparticle size via a simple mixing ratio. Hence, crystal quality, oxygen stoichiome-try, and defect density are constant throughout the sample and changes in the magnetic properties can be directly related to size.
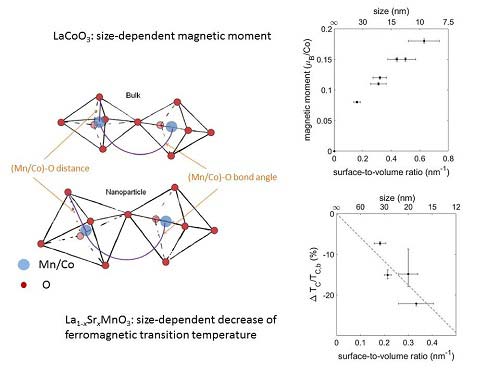
Decreasing nanoparticle size i.e. increasing surface-to-volume ratio leads to an elongation of the Mn/Co‑O bond length and a reduction of the Mn/Co‑O bond angle (not to scale). These changes result in a size-dependent increase of the magnetic moment in LaCoO3 and a size-dependent decrease of the ferromagnetic transition temperature in La1-xSrxMnO3
Cornelia E. Hintze
Karlsruhe Institute of TechnologySupervised by

Hilbert von Löhneysen
PhysicsHector Fellow since 2011