Defining novel resilience pathways in rare monogenic disorders
Daniel Petersheim — Hector Fellow Christoph Klein
In the EU alone, approximately 30 million people are affected by a rare disease, many of them children. Most of the 6,000 to 8,000 rare diseases known to date are caused by the altered function of a single gene (Boycott&Ardigó, 2018). This project under the supervision of Prof. Christoph Klein aims to develop innovative strategies for precision medicine in rare diseases by (i) re-wiring aberrant molecular networks for therapeutic purposes and (ii) identifying novel “druggable” targets using CRISPR-Cas9-mediated genome-wide screens.
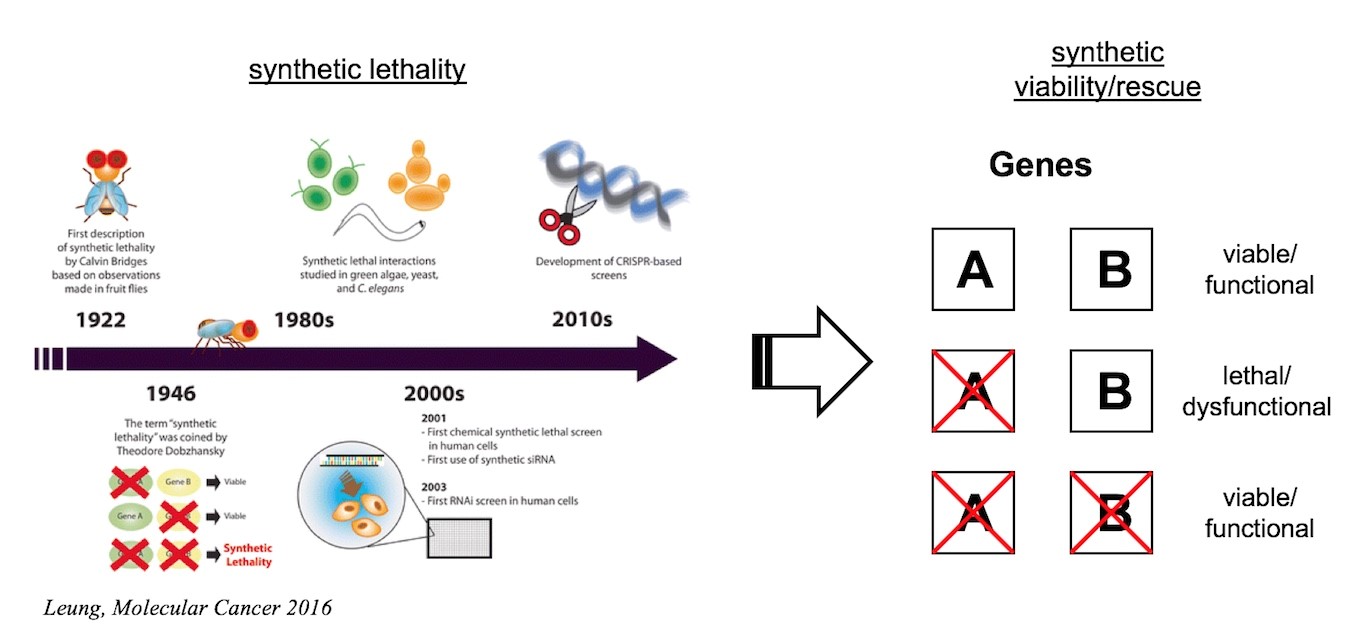
Synthetic lethality, a concept originally introduced by Dobzhansky in the context of his studies on Drosophila, describes a genetic interaction where the co-occurrence of two independent genetic events results in cell death. Conversely, synthetic rescue (synthetic viability) occurs when a cellular phenotype, which is due to the presence of a genetic mutation, can be reversed when the original mutation is combined with a second mutation affecting a different gene.
In medicine, the concept of synthetic lethality has fertilized the search for anti-cancer drugs and led to the discovery of PARP inhibitors. Whereas the power of CRISPR-Cas-mediated genome interrogations has been recognized in cancer, their enormous potential for patients with rare diseases remains to be explored. Like in cancer cells, genome-wide searches for pathways modulating the phenotype of cells carrying specific mutations holds great potential. In this project we will make use of CRISPR-Cas-based forward genetic screens to search for genes and pathways that may counteract deleterious consequences of rare disease-causing mutations and to re-wire aberrant networks for therapeutic purposes. While this strategy can be applied to a myriad of defined monogenic mutations in numerous defined cellular contexts, initial studies will make use of the near-haploid cell line KBM‑7 and focus on genetic defects associated with congenital neutropenia syndromes. Subsequent studies involving patient-derived iPS-cells will explore the clinical potential of the Approach.

Daniel Petersheim
Ludwig-Maximilians-Universität MunichSupervised by

Christoph Klein
Medicine & BiologyHector Fellow since 2013