Multidimensional Modeling of Inborn Errors of Hematopoiesis in a new three-dimensional Human Bone Marrow Organoid Model System
Megha Varghese Mukherjee – Hector Fellow Christoph Klein
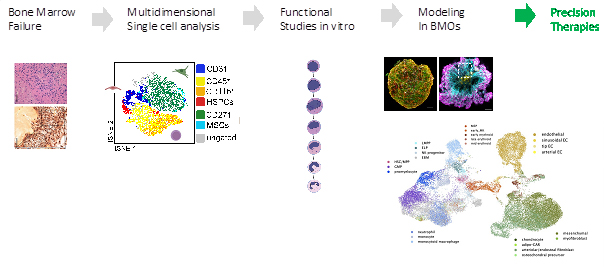
Rare genetic disorders lead to a failure to produce enough blood cells that are frequently fatal, seen most often among young children. These diseases are primarily monogenic, caused by the loss of function in a single gene. To investigate the effects of this loss of function, my project seeks to mimic it outside of the human body, specifically in human bone marrow organoids (BMOs). By studying BMOs, the aim is to identify critical factors contributing to bone marrow failure and ultimately use this information to develop new diagnostic methods.
All blood cells originate from hematopoietic stem cells (HSCs) in the bone marrow, a process known as hematopoiesis. HSCs can differentiate into both myeloid and lymphoid cells, giving rise to all blood cell types, and can undergo self-renewal and differentiation to increase their numbers. Hematopoiesis is a hierarchical and highly regulated process, and many genetic diseases are a result of dysfunction in this regulation, broadly termed bone marrow failure. However, studying these diseases has been challenging due to the limited availability of human bone marrow samples. Dysfunction in the bone marrow has proven difficult to study in animal models. Using human bone marrow organoids grown from iPSCs will allow these errors to be modeled in a human setting.
Severe congenital neutropenia (SCN) is a genetic disorder affecting hematopoiesis, with multiple genes contributing to or causing the disease. Deficiencies in VPS45, SMARCD2, HAX1, and ELANE lead to congenital neutropenia. By using CRISPR-Cas9 to introduce loss-of-function mutations in these genes, we can investigate whether bone marrow organoids can mimic the phenotype of these errors. I will determine if they can be used to investigate aberrations like fibrosis in these errors, by inhibiting defined pro-fibrinogenic pathways, aiming to elucidate the critical factors that lead to bone marrow dysfunction. Finally, I will establish how to translate this multi-omics single-cell-based analysis into cutting-edge diagnostics. My Ph.D. project aims to correlate morphological data on primary bone marrow smears and bone marrow organoids (BMOs) with genomic and transcriptomic datasets.
Human iPSC-derived bone marrow organoids – modeling errors in hematopoiesis

Megha Varghese Mukherjee
Ludwig-Maximilians-Universität MünchenSupervised by

Christoph Klein
Medicine & BiologyHector Fellow since 2013